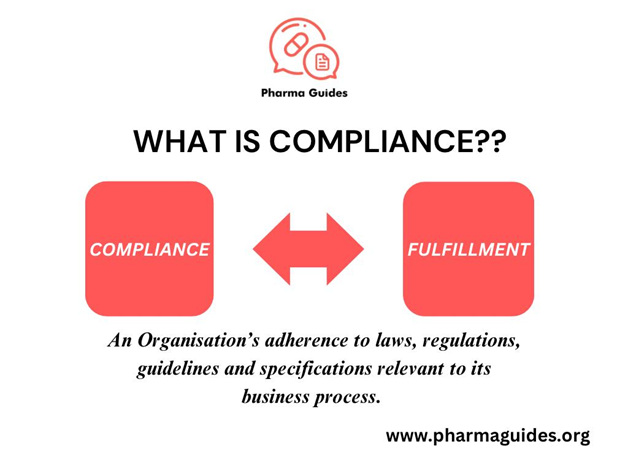
Compliance in the pharmaceutical industry means following rules and guidelines set by regulatory authorities like the FDA and EMA. It ensures safe, effective, and ethical manufacturing and distribution of drugs.
Key aspects include Good Manufacturing Practices (GMP) for consistent quality. Good Clinical Practice (GCP) protects human rights in trials. Good Laboratory Practices (GLP) ensure study quality and integrity.
Pharmacovigilance monitors post-marketing product safety. Regulatory Affairs ensures compliance with laws. Quality Management Systems (QMS) maintain quality standards.
Compliance is vital for public health and trust. Non-compliance can lead to sanctions and reputational damage. Pharmaceutical companies prioritize compliance and quality management to meet standards.
