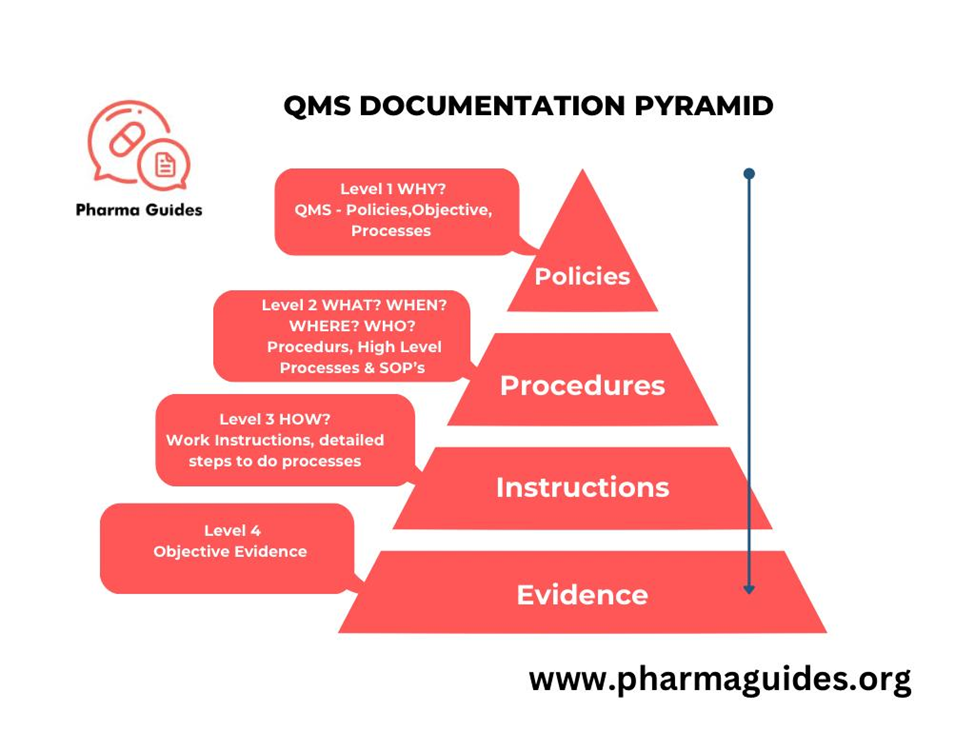
In the pharmaceutical industry, QMS Pyramid is structured in a hierarchical manner to ensure comprehensive and effective quality control and regulatory compliance. The levels of QMS documentation typically include the following:
Quality Manual:
Purpose: This is the highest-level document that outlines the company’s quality policy, objectives, and the overall structure of the QMS.
Content: It includes a description of the QMS, the scope of the system, key quality objectives, and the commitment to compliance with regulatory requirements.
Standard Operating Procedures (SOPs):
Purpose: SOPs provide detailed, written instructions to achieve uniformity in the performance of specific functions.
Content: These documents describe the procedures to be followed to ensure that operations are carried out consistently and in compliance with regulatory and quality standards. They cover various processes such as manufacturing, testing, documentation, and training.
Work Instructions:
Purpose: These are more detailed documents than SOPs, providing step-by-step instructions for specific tasks or operations.
Content: Work instructions include detailed descriptions of how to perform a particular task, often accompanied by diagrams or flowcharts to clarify complex steps.
Forms and Templates:
Purpose: These documents are used to record data, observations, and other information as required by SOPs and work instructions.
Content: Forms and templates standardize the documentation process, ensuring consistency and completeness in data recording. Examples include batch records, test result forms, and deviation reports.
Records:
Purpose: Records provide evidence of compliance with SOPs, work instructions, and regulatory requirements.
Content: These documents capture the results of various activities and include completed forms, logs, batch production records, and equipment maintenance logs.
Policies and Guidelines:
Purpose: These documents provide overarching principles and guidance to support the QMS.
Content: Policies define the company’s approach to quality and compliance, while guidelines offer best practices and additional details to support the implementation of policies and procedures.
