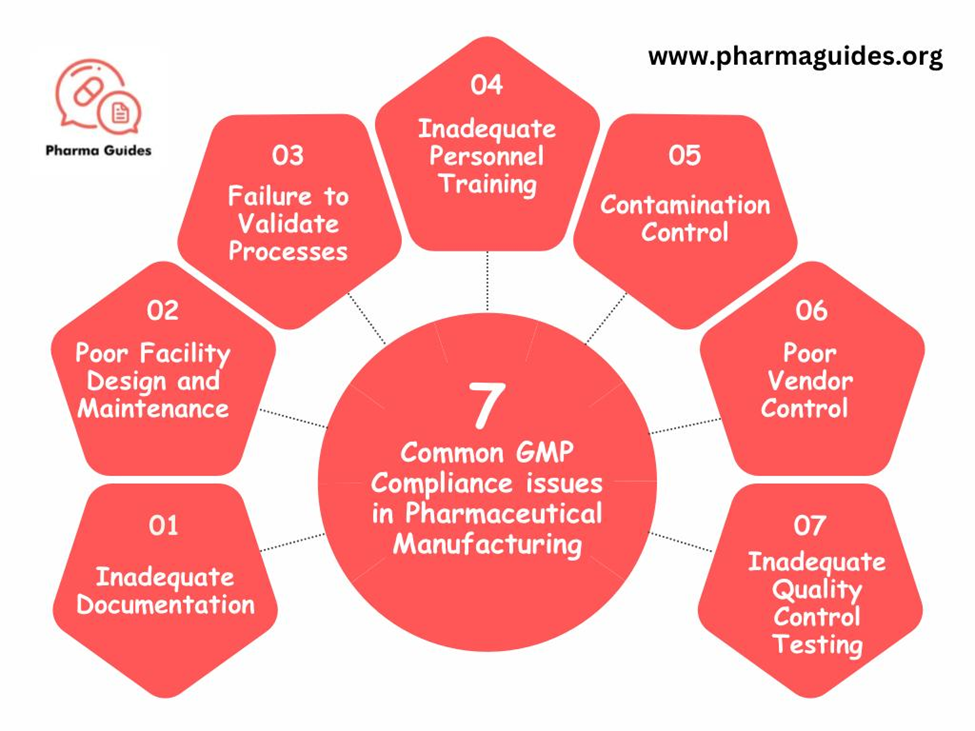
Ensuring Good Manufacturing Practices (GMP) compliance is crucial in pharmaceutical manufacturing. It maintains product quality, safety, and effectiveness. Here are 7 GMP issue:
- Inadequate Documentation: Often, documents are incomplete or wrong. This includes batch records, SOPs, and validation protocols. This leads to inconsistencies in processes and quality tracking issues.
- Poor Equipment Maintenance: Neglecting equipment leads to quality issues. Regular maintenance, calibration, and validation are crucial.
- Insufficient Personnel Training: Lacking GMP training leads to errors. Proper training ensures employees follow procedures, reducing violations.
- Contamination Control: Poor cleaning, facility design, or monitoring can contaminate products, compromising safety.
- Failure to Investigate Properly: Quick and full investigations are key to solving errors. Ignoring issues causes repeated problems and non-compliance.
- Supplier Issues: Using unqualified suppliers risks quality. Thorough evaluations and a strong qualification program are essential.
- Lack of Change Control: Changes without proper control can affect quality and compliance.
Addressing these issues needs a comprehensive approach. It includes quality management, training, audits, and a commitment to improvement. Pharmaceutical makers must prioritize GMP to ensure safe, high-quality medicines.
